Histology is the study of the microscopic anatomy of cells and tissues of plants and animals. It is commonly performed by examining cells and tissues under a light microscope, which have been sectioned, stained and mounted on a microscope slide. Histological studies may be conducted using tissue culture, where live human or animal cells are isolated and maintained in an artificial environment for various research projects. The ability to visualize or differentially identify microscopic structures is frequently enhanced through the use of histological stains. Histology is an essential tool of biology and medicine.
- The branch of biology dealing with the study of animal or plant tissues.
- The structure, especially the microscopic structure, of organic tissues.
- The study of the microscopic structure, chemical composition and function of the tissue or tissue systems of plants and animals.
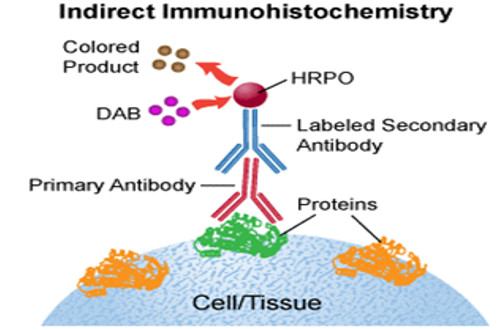
Immunohistochemistry or IHC refers to the process of detecting antigens (e.g., proteins) in cells of a tissue section by exploiting the principle of antibodies binding specifically to antigens in biological tissues.
Services:
The DARIS Center “Histology and Immunohistochemistry Unit” staff regarded the training and career development of staff, research groups, and students as essential in our mission to support the community. It offers routine processing for all types of fixed tissue for paraffin-embedded histology, Haematoxyline and Eosin (H+E) staining and special histology stains. While a Finesse microtome allows consistent sectioning quality.
Our aim is to continue to attract enthusiastic junior scientists and technicians just starting out on their careers to work with our established staff, to draw on their experience but also to spark new ideas in a stimulating research and teaching environment.
The Histology and Immunohistochemistry Lab is equipped with tissue processing machine, paraffin embedding center, semi-automatic microtome, paraffin section floatation bath and microscopes that can be used in a wide range of applications for samples preparation and screening. The facilities provide an excellent training environment, and each area has state-of-the-art equipment.
Applications of Histology:
- Education - Histology slides are often used in teaching laboratories to help students learn about the microstructures of human, animal and plant biological tissues.
- Diagnosis for treatment - Biological tissue samples taken from a patient (that is, a specific person or animal's body) may be studied in detail to enable medical or veterinary experts to learn more about the patient's condition and hence perhaps understand its causes and make recommendations for treatment or management of the condition.
Note: Although the study of the microstructure of diseased cells and tissues (e.g. to help inform decisions about treatment in clinical medicine) is an aspect or use of histology because it uses histological techniques, study of diseased tissues is more accurately called histopathology. - Forensic investigations- Forensic histology, immunohistochemistry and cytology involving microscopic study of biological tissues using various stains can help clarify the cause of sudden unexpected deaths and other issues in forensic science.
- Autopsy- Biological tissues from a deceased person or animal can be studied using histological techniques enabling experts to learn about the circumstances and possibly cause of death.
- Archaeology - Study of biological cells and tissues recovered from archaeological sites can provide information about history, even ancient history. The state of preservation of the biological material is critical, yet sometimes sufficient e.g. for bone histology and dental histology.
Key Stages in Preparing Histology Slides
The five main stages in the preparation of histology slides are:
1. Fixing:
This process has two phases:
1) The coagulation or precipitation of the various components of the tissues and cells.
2) Their preservation in a state as nearly as possible like the living condition by forming stable chemical compounds.
The specimen is placed in a liquid fixing agent (chemical fixative) such as formaldehyde solution (formalin). This will slowly penetrate the tissue causing chemical and physical changes that will harden and preserve the tissue and protect it against subsequent processing steps. There are a limited number of reagents that can be used for fixation as they must possess particular properties that make them suitable for this purpose. Fixation time, generally this will mean that the specimen should fix for between 6 and 24 hours.
*The most common fixative for light microscopy is 10% neutral buffered formalin (4% formaldehyde in phosphate buffered saline).
2. Processing:
Tissue processing is done to remove water from the biological tissues, replacing such water with a medium that solidifies, setting very hard and so allowing extremely thin sections to be sliced. This is important because biological tissue must be supported in an extremely hard solid matrix to enable sufficiently thin sections to be cut (5 μm thick for light microscopy).For light microscopy, paraffin wax is most frequently used. Since it is immiscible with water, the main constituent of biological tissue, water must first be removed in the process of dehydration. Samples are transferred through baths of progressively more concentrated ethanol to remove the water. This is followed by a hydrophobic clearing agent (such as xylene) to remove the alcohol, and finally molten paraffin wax, the infiltration agent, which replaces the xylene.
3. Embedding:
After the tissues have been dehydrated, cleared, and infiltrated with the embedding material, they are ready for external embedding. During this process the tissue samples are placed into molds along with liquid embedding material (Paraffin wax) which is then hardened by cooling. The hardened blocks containing the tissue samples are then ready to be sectioned. Because Formalin-fixed, paraffin-embedded tissues may be stored indefinitely at room temperature, and nucleic acids (both DNA and RNA) may be recovered from them decades after fixation. Formalin-fixed, paraffin-embedded tissues are an important resource for historical studies in medicine.
4. Sectioning
For light microscopy, a steel knife mounted in a microtome is used to cut (4μm -10μm -thick tissue sections which are mounted on a glass microscope slide. Then the mounted sections are treated with the appropriate stain. Sections can be cut through the tissue in a number of directions.
Possible orientations at which tissue samples may be sectioned include:
- Vertical sectioning perpendicular (at right-angles) to the surface of the tissue.
This is the most common method. - Horizontal sectioning is often done for the study of hair follicles and structures that include hairs, hair follicles, arrector pili muscles, and sebaceous glands in general. Such structures are sometimes called "pilosebaceous units".
- Tangential to horizontal sectioning is done in chemosurgery (also called "Mohs surgery") which is a form of microscopically controlled surgery used to treat certain types of skin cancer.
5. Staining:
Finally, the mounted sections are treated with an appropriate histology stain. Biological tissue has little inherent contrast in either the light or electron microscope. Staining is employed to give contrast to the tissue as well as highlighting particular features of interest. Where the underlying mechanistic chemistry of staining is understood, the term histochemistry is used. Haematoxyline and eosin (H&E stain) is the most commonly used light microscopical stain in histology and histopathology. Haematoxyline, a basic dye, stains nuclei blue due to an affinity to nucleic acids in the cell nucleus; eosin, an acidic dye, stains the cytoplasm pink.
Common laboratory stains
|
Stain |
Common use |
Nucleus |
Cytoplasm |
Red blood cell (RBC) |
Collagen fibers |
Specifically stains |
|---|---|---|---|---|---|---|
|
Haematoxyline |
General staining when paired with eosin (i.e. H&E) |
Orange, Cyan Blue or Green |
Blue/Brown/Black |
N/A |
N/A |
Nucleic acids—blue |
|
Eosin |
General staining when paired with Haematoxyline (i.e. H&E) |
N/A |
Pink |
Orange/red |
Pink |
Elastic fibers—pink |
|
Toluidine blue |
General staining |
Blue |
Blue |
Blue |
|
Mast cells granules—purple |
|
Masson's trichrome stain |
Connective tissue |
Black |
Red/pink |
Red |
Blue/green |
Cartilage—blue/green |
|
Mallory's trichrome stain |
Connective tissue |
Red |
Pale red |
Orange |
Deep blue |
Keratin—orange Cartilage—blue |
|
Weigert's elastic stain |
Elastic fibers |
Blue/black |
N/A |
N/A |
N/A |
Elastic fibers—blue/black |
|
Heidenhain's AZAN trichrome stain |
Distinguishing cells from extracellular components |
Red/purple |
Pink |
Red |
Blue |
Muscle fibers—red |
|
Silver staining |
Reticular fibers, nerve fibers, fungi |
N/A |
N/A |
N/A |
N/A |
Reticular fibers—brown/black |
|
Wright's stain |
Blood cells |
Bluish/purple |
Bluish/gray |
Red/pink |
N/A |
Neutrophil granules—purple/pink |
|
Orcein stain |
Elastic fibers |
Deep blue |
N/A |
Bright red |
Pink |
Elastic fibers—dark brown |
|
Periodic acid–Schiff (PAS) |
Basement membrane, localizing carbohydrates |
Blue |
N/A |
N/A |
Pink |
Glycogen and other carbohydrates—magenta |
Equipment:
1. AMOS ATP104 TISSUE PROCESSOR This tissue processer has two hanging basket (each basket can hold 72 standard embedding cassettes), and can run two program at one time. The processer uses CPU microcomputer control system, UPS device for protection from power cut and LCD. Tissue Processor
2. Tissue Embedding Center The Tissue Embedding Center (TEC) is a single module unit for moderate to heavy workloads in the preparation of wax tissue blocks. Features include microprocessor control of the large 4-liter paraffin reservoir, base molds warming oven, tissue holding tank, work stage and cold plate; user-friendly touch membrane pad with LED displays; lighted work stage; built-in forceps warmer; foot switch and/or push button-activated paraffin dispenser; and programmable, automatic timer controls. Tissue Embedding Center
3. Paraffin Section Floatation Bath
The Paraffin Section Flotation Bath is designed to assist with the handling of paraffin wax samples in histology and pathology laboratories. It is essentially, a hot distilled water floating out bath that allows for the meticulous manipulation and location of sections onto glass slides.

4. Semi-Auto Microtome
A microtome is a tool used to cut extremely thin slices (2µm -10µm) of material, known as sections. Important in science, microtomesare used in microscopy, allowing for the preparation of samples for observation under transmitted light radiation.

Semi-Auto Microtome
5. Slides Warmer
Compact slide warmer with digital temperature control and LED readout is ideal for quickly drying slides for the histology, pathology and life science research lab.

Slides Warmer